
The government's Green Book has the latest information on vaccines and vaccination procedures, for vaccine preventable infectious diseases in the UK - including 'informed' consent.
Compare tables for COVID19 (chapter 14a) and Flu (chapter 19) for different ages and at risk groups.
Front cover and Index January 2021 (PDF)
Update 28FEB22
Part 1: Principles, practices and procedures
- Chapter 1: Immunity and how vaccines work (JAN21)
- Chapter 2: Consent (18JUN21)
- "It is a legal and ethical principle that valid consent must be obtained before starting personal care, treatment or investigations. This refects the rights of individuals to decide what happens to their own bodies and consent is a fundamental principle of good healthcare and professional practice. ... For consent to immunisation to the valid, it must be given freely, voluntarily and without coercion by an appropriately informed person who has the mental capacity to consent to the administration of the vaccines in question ... The exchange of information between the healthcare provider and the individual is key to ensuring informed consent"
- Chapter 3: Storage, distribution and disposal of vaccines
- Chapter 4: Immunisation procedures (20MAR13)
- Chapter 5: Immunisation by nurses and other health professionals
- Chapter 6: Contraindications and special considerations
- Chapter 7: Immunisation of individuals with underlying medical conditions
- Chapter 8: Vaccine safety and adverse events following immunisation (20MAR13)
- Chapter 9: Surveillance and monitoring for vaccine safety (20MAR13)
- "Important information on vaccine safety is routinely collected through the Yellow Card scheme and from other sources ..."
- Chapter 10: Vaccine Damage Payment Scheme (20DEC18 - Blog)
- Chapter 11: Immunisation schedule (02JAN20)
- Chapter 12: Immunisation of healthcare and laboratory staff
Part 2: The diseases, vaccinations and vaccines
- Chapter 13: Anthrax: chapter 13
- Chapter 14: Cholera:
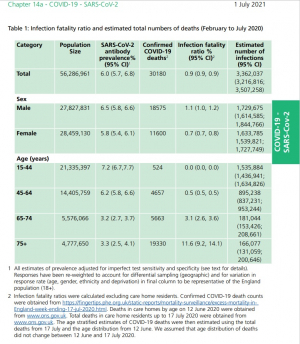
- Chapter 14a: COVID-19 - SARS-CoV-2:chaper 14a (01JUL21)
- "There is currently limited evidence to support the use of COVID-19 vaccines as post-exposure prophylaxis or to interrupt transmission during outbreaks. The use of vaccine to provide direct protection to vulnerable individuals in prolonged community outbreaks should be discussed with the local health protection teams ...
- Children and young people have a very low risk of COVID-19, severe disease or death due to SARS-CoV-2 compared to adults and so COVID-19 vaccines are not routinely recommended for children and young people under 16 years of age ...
- As with most pharmaceutical products, large clinical trials of COVID-19 vaccine in pregnancy have not been carried out ... Clinicians should discuss the risks and benefits of vaccination with the woman, who should be told about the limited evidence of safety for the vaccine in pregnancy ... (see below)
- As there is limited evidence on response in immunosuppressed individuals there is also very little evidence upon which to base advice on the optimal timing of delivery
- Individuals who experience severe expected reactions after a first dose of AstraZeneca or Pfizer vaccines appear to have a higher rate of such reactions when they receive a second dose of the alternate vaccine.
- ... no data for co-administration of COVID-19 vaccine with other vaccines exists... Studies are on-going to support co-administration of COVID-19 vaccines with influenza in the 2021-2022 season. Where co-administration does occur, patients should be informed about the likely timing of potential adverse events relating to each vaccine.
- Anaphylaxis following COVID-19 vaccination ... the MHRA is no longer advising that individuals with a history of anaphylaxis to any vaccine, medicine or food do not get the vaccine. Anyone with a previous history of allergic reactions to the ingredients of the vaccine should not receive it, but those with any other allergies (such as a food or penicillin allergy) can have the vaccine."
- ...
- Chapter 19: Infuenza: chapter 19 (29OCT20)
- ....
- Chapter 35: Yellow fever: chapter 35
- The complete 2006 edition of The Green Book is available via The National Archives. This does not contain the latest chapter updates (associated letter)
See also:
- 18JUN21 Mandatory Vaccination for Social Care Workers
- "Government will now bring forward Regulations that will require all CQC regulated service providers of nursing and personal care, in care homes in England, to allow entry to the premises only those who can demonstrate evidence of having had a complete course of an authorised Covid-19 vaccine (or evidence that they are exempt from vaccination) ...the policy will also apply to all persons who enter a care home, regardless of their role
- "Any employee dismissed because they because they have not been vaccinated may potentially have a claim for unfair dismissal. We would have to see the detail of the case and also the legal instrument the Government employs to bring in mandatory vaccination in the care sector." (PDF)
- 18JUN21 Inadvertent vaccination in pregnancy
- "There are no known risks for women who are vaccinated against COVID-19 or measles, mumps, rubella, chickenpox or shingles during any stage of pregnancy or shortly before conception"
- "If you are pregnant and have chosen to be vaccinated and would like to report your vaccination details, you can do so by registering with the Vaccine Monitor run by the Medicines and Healthcare products Regulatory Agency (MHRA) before or around the time of vaccination. Please select the Yellow Card Vaccine Monitor – invitation to pregnant women"
- MHRA Please help monitor COVID-19 vaccine safety in pregnancy (PDF)
- "The large trials which showed that these vaccines are safe and effective did not include pregnant women – as often happens in clinical trials" (Also see above).

- 16JUN21 Ill thought through plans to mandate vaccinations could lead to care staff 'exodus' (PDF)
- "More than a third of our GMB Union carers would consider packing their jobs in if vaccines were mandated" (Young people have concerns as being child bearing age 3min video)"
- 16JUN21 Making vaccination a condition of deployment in care homes: government response (PDF)
- "DHSC undertook thorough analysis of the more than 13,500 consultation responses and considered the feedback received. Overall, the consultation showed that, while a majority (57%) of respondents did not support the proposal, the responses from the adult social care sector were mixed, with some group, for example care home providers mostly supporting the proposed legislative change while others, such as service users and relatives of service users were mostly opposed. ... initial proposals set out that individuals will be exempt from the requirement if they have an allergy or condition that the Green Book lists (COVID-19: the green book, chapter 14a)" Extract Diagram1 and Diagram2
Extract Diagram1
Extract Diagram2
- "DHSC undertook thorough analysis of the more than 13,500 consultation responses and considered the feedback received. Overall, the consultation showed that, while a majority (57%) of respondents did not support the proposal, the responses from the adult social care sector were mixed, with some group, for example care home providers mostly supporting the proposed legislative change while others, such as service users and relatives of service users were mostly opposed. ... initial proposals set out that individuals will be exempt from the requirement if they have an allergy or condition that the Green Book lists (COVID-19: the green book, chapter 14a)" Extract Diagram1 and Diagram2
- 30APR21 The safety of COVID-19 vaccines when given in pregnancy (PDF)
- 25FEB21 GMB Union Vaccination Notice (PDF)
- "we understand that some of our members are not able to be vaccinated e.g. for health reasons or pregnancy"
- PHE Excess mortality in England per week - Experimental Statistics (26FEB21 & 17JUL20)







Make A Comment
Comments (0)